.
Phytochemical
rich food studies and prostate cancer
This page describes the previous and
ongoing studies which have evaluated phytochemical rich food supplements,
including the latest double blind national trial, which started in September
2023, is called the YourPhyto study which has been featured first as it is
the most current. Other prospective cohort studies are also summarised on
this page:
-
The YourPhyto Study >>
-
The Pomi-T trial >>
-
The PSA-MRI
correlation Study >>
-
The Tokyo Women's
study >>
-
The Tea and prostate
cancer cohort study >>
-
The broccoli and
prostate cancer study >>
.
 The
Your Phyto Study The
Your Phyto Study
This
study evaluated the next generation of phytochemical rich supplements
in combination with a probiotic vitamin D complex. It was a national,
ethically approved RCT started in September 2023 and finished in June
2024. The initial results were presented at ASCO
Urology 2025
It aimed to establish whether boosting the diet with a lactobacillus probiotic (Yourgutplus)
in addition to a phytochemical-rich food supplement (Yourphyto)
influenced PSA progression, prostate-related symptoms and strength in
men with early PCa compared to placebo.
The probiotic capsule contained 10 billion colony forming units (CFU) of 5 lactobacillus strains
with built-in prebiotics. The phytochemical-rich capsule will
contain whole foods which have previously reported potential benefits for
men with prostate cancer in epidemiological, laboratory and prospective
studies. The ingredients of both supplements have been shown to have a
high safety profile.
Cohort: Men
with histologically proven, early PCa early not taking androgen
deprivation therapy (ADT), managed with active surveillance or watchful
waiting.
 Method:
Following
written informed consent, all participating men (220) will be given the
phytochemical-rich food supplement and asked to stop all other
over-the-counter supplements (except vitamin
D). This was a double-blind, randomised
(1:1) allocation of the probiotic supplement or placebo. The
supplements were taken twice a day fo Method:
Following
written informed consent, all participating men (220) will be given the
phytochemical-rich food supplement and asked to stop all other
over-the-counter supplements (except vitamin
D). This was a double-blind, randomised
(1:1) allocation of the probiotic supplement or placebo. The
supplements were taken twice a day fo
Results: The rate of rise of Prostatic Specific
Antigen (PSA) significantly slowed (more than three fold) if men took
YourPhyto only (This was a much higher level than seen in the similar
study in 2013). In men who were also randomised to received the additional
probiotic (Yourgutplus) PSA progression reduced by 43% (in effec, this
meant that most men's PSA actually feel) . There was a high patient safety
with no significant adverse event. Uniquely the study measured markers of
wellbeing such a prostate symptoms:
Clinical implications: More men were
reassured to safely stay on active Surveillance with the support of these
two supplements. This trial provides more information on
phytochemical rich foods and strategies to improve gut health. Most men,
who take these sort of supplements are now switching to yourphyto, in view
of these positive trial results.
Sponsors: The study was sponsored by
Bedford and Luton Hospital trusts and the supplement have been supplied,
at no cost to the trials unit, from the manufactures, who had (or will
have) no influence in the trial design conduct, analysis or publication of
the study
For more information: Please
refer to the Yourphyto
website
|
|
The
Pomi-T Study: A randomised
double blind placebo
controlled trial of a polyphenol
rich dietary food supplement in men with prostate
cancer
|
This randomised controlled trial has now completed. The results were
announced as a full oral presentation at the world's largest and most
prestigious cancer conference ASCO in 2013. The full
paper was published in a Nature Journal in 2014. In 2016, a further trial
was conducted and published which confirmed the very low progression of PSA in
men taking Pomi-T on surveillance but also confirmed that this correlated with
underlying control of disease seen on MRI (read second paper).
The supplement used in
this trial had the highest practicable quality assurance, including specific
test for authenticity, purify and lack of heavy metal or bacterial
contamination of the ingredients. In addition, an independent academic body
performed mass spectrometry to ensure no unexpected additives were present.
The original manufacturers agreed with the clinical trials committee to
continue this high quality assurance programme but unfortunately unregulated
copies have since appeared on some UK and international websites. If you are
interested in taking Pomi-T we recommend
you refer to the original regulated website and avoid discount stores which
may not adhere to the same quality assurance.-
link to Pomi-T website.
Background to the study
An increasing numbers of published studies have linked polyphenols, the
natural plant based phytochemicals found in healthy foods, with a lower risk of
chronic illnesses such as dementia, arthritis, skin aging, macular degeneration
and more recently cancer [Rezai-Zadeh], [Maclarty]. Women with early breast
cancer taking higher than the recommended “5 a day” amount of fruits and
vegetable have been found to lower their risk of recurrence risk by one third
especially if combined with physically activity [Pierce 2009]. Breast cancer
recurrence has been demonstrated to be lower amoung women regularly drinking
green tea [Ogunleye 2010] or eating foods rich in dietary lignans [Buck 2011],
isoflavones and flavanones [Boyapati 2005]. A full scientific review of the
evidence for polyphenols and cancer has been conducted by the trials team and
can be downloaded free.
Although the benefits of boosting the diet with polyphenol rich whole food
supplements for individuals with cancer have been investigated in small phase II
studies, they have rarely been evaluated within an adequately powered Randomised
Controlled Trials. For this reason, the UK’s government’s clinical trials
design committee decided to set up a national trial to investigate whether there
are any anticancer benefits. These committee green tea, broccoli, turmeric and
pomegranate of chose these foods as they originate from separate categories
(spice, herb, fruit, vegetable), hypothesising that their diverse polyphenol
profile would have synergistic action whilst avoid accumulation of one
particular phytochemical. Each ingredient has also demonstrated anti-oxidant
activity, protecting cells from carcinogenic exposure, as well as direct anti-neoplastic
activity within previous laboratory or phase II trials:
-
 Green
Tea, rich in epigallocatechin gallate, blocks ornithine decarboxylase
resulting in reduced proliferation, angiogenesis and de-differentiation in
cancer cell lines. A meta-analysis of 5000 women showed that regular consumption
had reduced breast cancer recurrence. Phase 2 studies have demonstrated reduced
PSA in prostate cancer [Mclarty, Ogunleye Porrini, Liao]. Green
Tea, rich in epigallocatechin gallate, blocks ornithine decarboxylase
resulting in reduced proliferation, angiogenesis and de-differentiation in
cancer cell lines. A meta-analysis of 5000 women showed that regular consumption
had reduced breast cancer recurrence. Phase 2 studies have demonstrated reduced
PSA in prostate cancer [Mclarty, Ogunleye Porrini, Liao].
-
Pomegranate, rich in ellagic acid, which in cancer cell lines reduced
proliferation, apoptosis and adhesion. In 3 phase II studies it prolonged PSAdt
and reduced oxidative stress [Retitig, Lansky, Malik, Barber, Rocha, Wang,
Pantuck, Carducci, Paller].
-
Turmeric, rich in capsaicin reduced growth, invasion, migration, and
TK activation of EGFR. In cancer stem cells it prevented progression to
prostate, bowel and breast cancer but had no affect on normal stem cells. In
humans has demonstrated Cox-I anti- inflammation effects [Kakarla,
Shah, Zhang, Dorai]
-
Broccoli, rich in iothiocyanate, in cell lines slows growth and
promotes apoptosis. In humans after regular consumption it alters genetic
signature, down regulates promoting genes, up regulate cancer suppressor genes
[Gasper, Joseph, Heinen].
Methodology
 Two hundred and three men, aged 53-89 yrs (average 74 yrs), with prostate
cancer (95% Gleason 6/7, 5% >7), 59% managed with primary active
surveillance or 41% with watchful waiting (WW) with a progressive PSA relapse
following previous radical interventions were randomised to receive a Pomi-T
or an identical placebo for 6 months. The randomised process produced no
statistical difference in baseline characteristics except the placebo group
were slightly older which, if anything would be more advantageous to the
placebo group. The power calculation and independent statistical analysis took
place at Cranfield University and men were recruited from across the UK.
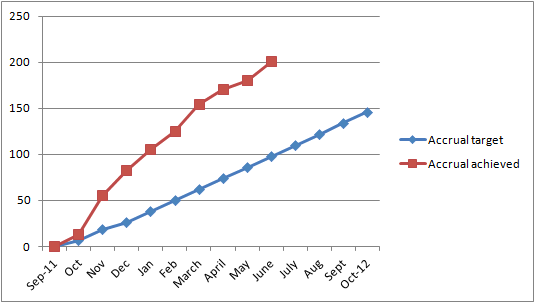
Enthusiasm to enter the study was overwhelming. The trials team completed the
quota 10 months ahead of schedule (see graph). Two hundred and three men, aged 53-89 yrs (average 74 yrs), with prostate
cancer (95% Gleason 6/7, 5% >7), 59% managed with primary active
surveillance or 41% with watchful waiting (WW) with a progressive PSA relapse
following previous radical interventions were randomised to receive a Pomi-T
or an identical placebo for 6 months. The randomised process produced no
statistical difference in baseline characteristics except the placebo group
were slightly older which, if anything would be more advantageous to the
placebo group. The power calculation and independent statistical analysis took
place at Cranfield University and men were recruited from across the UK.
Enthusiasm to enter the study was overwhelming. The trials team completed the
quota 10 months ahead of schedule (see graph).
 Quality
assurance: This non commercial academic trial received peer reviewed
sponsorship from Prostate Action, was designed by NCRI Complementary Therapies
Research Committee, adopted by the UK’s NCRN and independently audited to
ensure it adherence to European Good Clinical Practice Guidelines. The
manufacturers performed in house analysis to ensure authenticity and purity and
a further independent mass spectrometry was performed to confirm purity.
Statistical evaluation was independent to the trials unit. Quality
assurance: This non commercial academic trial received peer reviewed
sponsorship from Prostate Action, was designed by NCRI Complementary Therapies
Research Committee, adopted by the UK’s NCRN and independently audited to
ensure it adherence to European Good Clinical Practice Guidelines. The
manufacturers performed in house analysis to ensure authenticity and purity and
a further independent mass spectrometry was performed to confirm purity.
Statistical evaluation was independent to the trials unit.
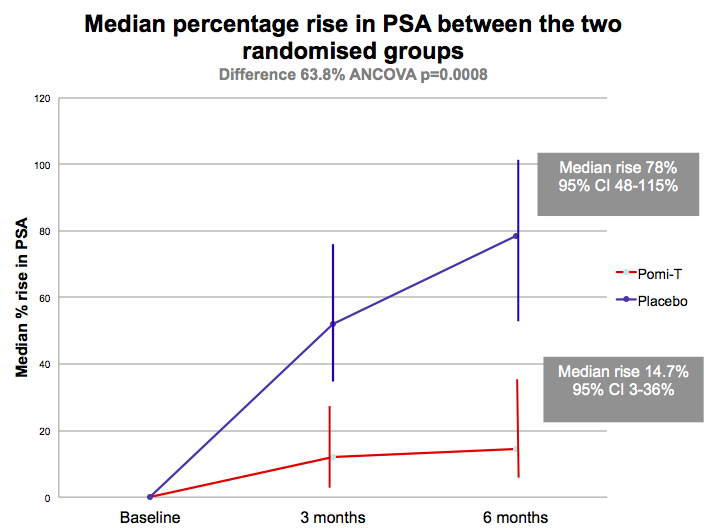
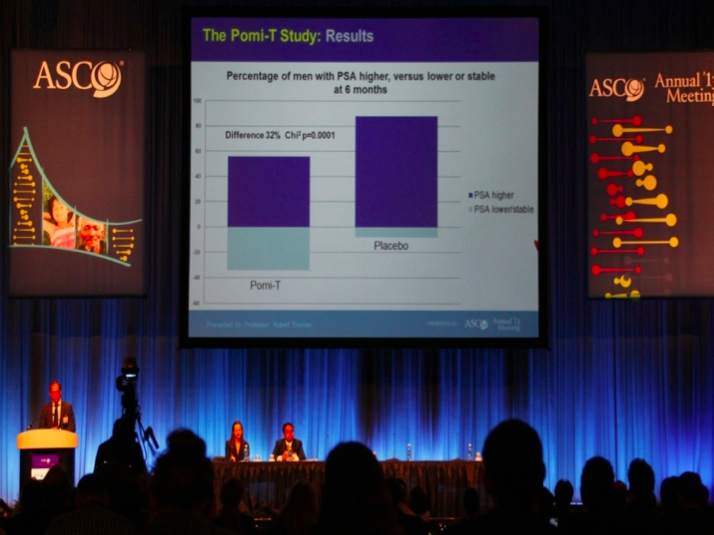
Results: Two men from each group men withdraw
before the first 3 month evaluation. of the remaining 199 men, the median
rise in PSA in the Pomi-T was 14.7% (95% CI 3.4-36.7%) v 78.5% in the PG (95% CI
48.1-115.5%). This difference of 63.8% was significant (ANCOVA p=0.0008). 18.6%
more men stayed off interventions at the end of the trial in the Pomi-T group.
There was no significant difference between any of the pre-determined
subgroups.
 Secondary end points: There was no effect on average serum sex hormone
analysis: Testosterone 13.4 nmol/l (nr 9-29) FSH 9.2 iu/l (nr 2-12), LH 7.4 iu/l
(nr 2-9) There was no effect on cholesterol, INR or BP amoung men taking
warfarin or ramipril. MRI scans, taken routinely as part of their AS protocol,
were retrospectively evaluated. 35% had no visual disease, 15% had progressive
disease with significant rises in PSA (removed from trial) 50% had visual
disease with no progression, no man remaining on Pomi-T had MRI defined
progression. There was a 14% difference in urinary symptoms (mainly
urgency) and a 6% difference in joint pains although these two endpoint were not
predetermined in the statistical plan so need further varification. Secondary end points: There was no effect on average serum sex hormone
analysis: Testosterone 13.4 nmol/l (nr 9-29) FSH 9.2 iu/l (nr 2-12), LH 7.4 iu/l
(nr 2-9) There was no effect on cholesterol, INR or BP amoung men taking
warfarin or ramipril. MRI scans, taken routinely as part of their AS protocol,
were retrospectively evaluated. 35% had no visual disease, 15% had progressive
disease with significant rises in PSA (removed from trial) 50% had visual
disease with no progression, no man remaining on Pomi-T had MRI defined
progression. There was a 14% difference in urinary symptoms (mainly
urgency) and a 6% difference in joint pains although these two endpoint were not
predetermined in the statistical plan so need further varification.
Conclusions:
In this cohort of men with prostate cancer managed with AS or WW this
study demonstrated 6 months of Pomi-T demonstrated highly statistically
significant short term favourable effect on the percentage rise in PSA compared
to placebo. It was well tolerated without any significant adverse effects or
concomitant drug interactions and resulted in significantly more men remaining
on AS or WW avoiding the toxicities and expense of medical interventions. No
change in testosterone levels occurred in men taking Pomi-T and disease seen on
MRI correlated with PSA dynamics.
The
PSA - MRI correlation study
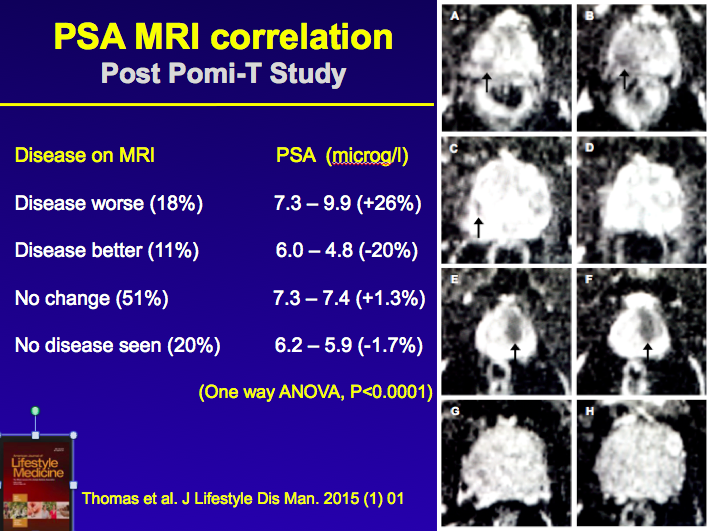 A second evaluation has taken place involving men who have continued to take
pomi-t post trial. It specifically addressed the issue of of whether PSA
correlated with underlying changes in Cancer size seen on MRI. It involved men
followed for up to 2 years on active surveillance. A second evaluation has taken place involving men who have continued to take
pomi-t post trial. It specifically addressed the issue of of whether PSA
correlated with underlying changes in Cancer size seen on MRI. It involved men
followed for up to 2 years on active surveillance.
The results, as shown in the adjacent picture, should a 100% correlation
between PSA and underlying disease indicating that the PSA effect on the main
Pomi-t trial was unlikely to be just a chemical effect but related to actual
prostate cancer itself - read full paper
The trials team has now designed a RCT evaluating a polyphenol
rich nail balm with the aim of reducing chemotherapy induced nail damage
this trial is underway - read more.
The team has also performed two evidence reviews summarising the evidence for polyphenols
and exercise. A
further evidence review examining the Biology of exercise has been write with
Coventry University and Stacey Kenfield from the University f South California
and been published in the British Journal of Sports medicine and is available to
read here.
The trials unit are moving on to evaluate the evidence for a polyphenol rich supplement to
improve arthritis and exercise levels. In the mean time they have
successfully designed and conducted a double blind randomised trial of a
polyphenol rich nail balm to stop distressing chemotherapy associated nail
damage. This trial known as the polybalm study has been published in ASCO
2017.... read more
The
Tokyo Women's study
The
effect of a boosting phytochemical rich
foods for women’s cancer survivors on arthralgia, mood and hot flushes.
Yanagisawa Y et al (2021). J Nurs Women’s Health 5: 168. DOI: 10.29011/2577-1450.100068
Background
and rationale:
 Late
toxicities such as arthritis, low
mood, nail
changes and hot
flushes are common after breast, other women’s cancers
and after menopause in general. As well as causing discomfort impacting
quality of life, persistent arthralgia can compromise the effectiveness
of adjuvant therapies by reducing compliance to hormone
treatment. Discontinuation rates of hormone therapies such as tamoxifen
and aromatase inhibitors have been recognised in up to 30% of women
suffering from arthralgia and stiffness. Poor adherence to AI therapy has also
been associated with worse disease-free and overall survival from breast
cancer. In addition, arthritis can significantly impact patients ability
to exercise,
which can lead to an exacerbation of further symptoms and
complications associated with cancer. In particular, regular
exercise can mitigate the risks of weight
gain, hot
flushes, fatigue,
peripheral neuropathy, erectile function, arthritis itself
and osteoporosis,
as well as improve mood and sleep
patterns. Restricted mobility may have even more sinister consequences for
patients, as cohort studies have consistently linked a reduced relapse rate
and improved survival benefit amongst those able to be physically active after
several different types of cancer, including breast cancer..... Late
toxicities such as arthritis, low
mood, nail
changes and hot
flushes are common after breast, other women’s cancers
and after menopause in general. As well as causing discomfort impacting
quality of life, persistent arthralgia can compromise the effectiveness
of adjuvant therapies by reducing compliance to hormone
treatment. Discontinuation rates of hormone therapies such as tamoxifen
and aromatase inhibitors have been recognised in up to 30% of women
suffering from arthralgia and stiffness. Poor adherence to AI therapy has also
been associated with worse disease-free and overall survival from breast
cancer. In addition, arthritis can significantly impact patients ability
to exercise,
which can lead to an exacerbation of further symptoms and
complications associated with cancer. In particular, regular
exercise can mitigate the risks of weight
gain, hot
flushes, fatigue,
peripheral neuropathy, erectile function, arthritis itself
and osteoporosis,
as well as improve mood and sleep
patterns. Restricted mobility may have even more sinister consequences for
patients, as cohort studies have consistently linked a reduced relapse rate
and improved survival benefit amongst those able to be physically active after
several different types of cancer, including breast cancer.....
 Epidemiology
studies have linked higher intake of polyphenol-rich foods with better gut
health mood and less arthritis. Lab studies show these
foods reduce intra-articular inflammation and
oxidative stress, allowing greater mobility although, up until now,
clinically relevant, intervention studies in humans have been lacking. A
whole food nutritional supplement can be a convenient way to boost intake of
polyphenol-rich foods and ensure sustained intake throughout the day.
For this study the OTC supplement Pomi-T was
selected given that it has demonstrated a high safety profile in a
randomised trial in which participants also reported improvements in joint
discomfort. Pomi-T also contains no phytoestrogenic polyphenols, which may
potentially would have been a concern post breast cancer... Epidemiology
studies have linked higher intake of polyphenol-rich foods with better gut
health mood and less arthritis. Lab studies show these
foods reduce intra-articular inflammation and
oxidative stress, allowing greater mobility although, up until now,
clinically relevant, intervention studies in humans have been lacking. A
whole food nutritional supplement can be a convenient way to boost intake of
polyphenol-rich foods and ensure sustained intake throughout the day.
For this study the OTC supplement Pomi-T was
selected given that it has demonstrated a high safety profile in a
randomised trial in which participants also reported improvements in joint
discomfort. Pomi-T also contains no phytoestrogenic polyphenols, which may
potentially would have been a concern post breast cancer...
How
was the study conducted:
This was an open label evaluation of a polyphenol-rich whole food
supplement (Pomi-T) supplied to volunteer members of a Japanese Women’s
Cancer Support Group, Tokyo. Joint discomfort, mobility, mood and hot flushes
were recorded at baseline and after 2 months Pomi-T using validated peer
reviewed questionnaires. 38 of 44 (87%) completed pre and post forms (average
age 48, range 26-62 years).
The
Pomi-T supplement consisted of a vegan capsule containing 150mg whole
pomegranate fruit powder (Punica granatum) ; 30mg ground green tea leaf
(Camellia sinensis) of 5:1 extract (equivalent to 150mg of whole leaf); 150mg
whole ground turmeric; 150mg whole ground broccoli florets (brassica oleracea).
The Swiss manufacturers (Helsinn Integrative) are adherent to good
manufacturing practice guidelines and perform in-house analysis for
authenticity and purity from heavy metals and pesticides on each batch. It was
supplied free to the women volunteers by the Japanese distributor PT Plus
(Higashi Kanamachi 5-48-28, Katsushika-ku, Tokyo 125-0041, Japan).
Results: There was a statistically significant improvement in mean mood scores
was 1.9 (19.96-18.06), with a paired T-test of p=0.01. Mean reduction in
hot flush score was 6.68 (38.41-31.71), with a two tailed sign test of p=
0.016. Mean reduction in joint pain, stiffness and immobility was 3.84
(25.21-21.37), with a two tailed sign test of p=0.011. In practical terms this
represented about a 10% improvement in symptoms. Positive comments from
individuals (Table.1) were received from 30 of 38 (79%) women and included an
unexpected reduction in hay fever symptoms in 8 (21%) of women.
Conclusion: This open labelled study demonstrated that symptomatic women with
breast and other had a statistically significant reduction in arthralgia, hot
flushes and improved mood after consuming two capsules of Pomi-T per day.
This evaluation did not formally measure hay fever symptoms, and the
comments from the participants were spontaneous. This was not completely
unexpected, as polyphenols have previously been investigated for their
anti-allergic effect in models and in human clinical trials [97, 98]. This
evaluation took place in late spring in Japan, just before the “Cherry
Blossom” season which is associated with a higher pollen count. It
would be interesting to repeat this evaluation focusing specifically on the
effect of Pomi-T in individuals who suffer from hay fever over a similar
time period using designated formal measures of allergy symptom severity.
Read more on the
Pomi-T website
|
References:
Barber ( 2006) Prost Can Pros Dis.9(4): 407-13.
Brasky (2011). Nutr Cancer. 63(4):573-82
Bauer (2012). Integr Can Ther. 2012 11(2):83-9.
Boyapati (2005) Breast Cancer Res T.92:11–7.
Buck (2011). JCO, 29 (28). 3730-38.
Carducci (2011). JCO, 29: 7, 11.
Chaoyang (2011) Arch Intern Med 171(6); 507-15.
Chuang (2011)EJC 47, 1808-16.
Clarke (2006) Urology 67 (6): 1257-61.
Heinonen (1998) J Nat Can Instit ; 90: 440-9
Gasper AV (2010) Molecular Cancer 2010, 9:189.
Giovannucci (2002) JN Can Instit, 94: 391-398.
Handler (2007). Chem Pharm Bull. 55(1): 64-71
Haris (2014) EJC 50, 1223-1231
|
Heinen (2007) EJC 43; (18) 2707-16.
Joseph (2004) Nutr Cancer, 50(2):206-213.
Kakarla (2010) Res treat 122(3):777-85
Malik (2005) Proc Natl Acad Sci USA.
McLarty (2009). Can Prev Res: 1940-6207.
Ogunleye (2010) Breast Cancer Res T 119(2):477.
Paller (2013) Prost Can & Prost Dis 16, 50-55.
Pantuck (2005) J Urol.
173:225–226.
Pierce (2007 JAMA 298(3): 289-98.
Porrini (2008) Nutr Metab Cardio:80(4):353-61. Shah (1999). Bio Pharm., 58(7):
1167–72.
Thomas (2013) JCO. 31, Suppl; 5008
Thomas (2014) PCPD (nature.com/pcan ) Jan 1-7
Tung (2005) Can Epi Biomarkers Prev; 14;669
Wang (2011). Integr Biol (Camb);3:742–754
|
|
|



 Green
Tea, rich in epigallocatechin gallate, blocks ornithine decarboxylase
resulting in reduced proliferation, angiogenesis and de-differentiation in
cancer cell lines. A meta-analysis of 5000 women showed that regular consumption
had reduced breast cancer recurrence. Phase 2 studies have demonstrated reduced
PSA in prostate cancer [Mclarty, Ogunleye Porrini, Liao].
Green
Tea, rich in epigallocatechin gallate, blocks ornithine decarboxylase
resulting in reduced proliferation, angiogenesis and de-differentiation in
cancer cell lines. A meta-analysis of 5000 women showed that regular consumption
had reduced breast cancer recurrence. Phase 2 studies have demonstrated reduced
PSA in prostate cancer [Mclarty, Ogunleye Porrini, Liao].

 Quality
assurance: This non commercial academic trial received peer reviewed
sponsorship from Prostate Action, was designed by NCRI Complementary Therapies
Research Committee, adopted by the UK’s NCRN and independently audited to
ensure it adherence to European Good Clinical Practice Guidelines. The
manufacturers performed in house analysis to ensure authenticity and purity and
a further independent mass spectrometry was performed to confirm purity.
Statistical evaluation was independent to the trials unit.
Quality
assurance: This non commercial academic trial received peer reviewed
sponsorship from Prostate Action, was designed by NCRI Complementary Therapies
Research Committee, adopted by the UK’s NCRN and independently audited to
ensure it adherence to European Good Clinical Practice Guidelines. The
manufacturers performed in house analysis to ensure authenticity and purity and
a further independent mass spectrometry was performed to confirm purity.
Statistical evaluation was independent to the trials unit.  Secondary end points: There was no effect on average serum sex hormone
analysis: Testosterone 13.4 nmol/l (nr 9-29) FSH 9.2 iu/l (nr 2-12), LH 7.4 iu/l
(nr 2-9) There was no effect on cholesterol, INR or BP amoung men taking
warfarin or ramipril. MRI scans, taken routinely as part of their AS protocol,
were retrospectively evaluated. 35% had no visual disease, 15% had progressive
disease with significant rises in PSA (removed from trial) 50% had visual
disease with no progression, no man remaining on Pomi-T had MRI defined
progression. There was a 14% difference in urinary symptoms (mainly
urgency) and a 6% difference in joint pains although these two endpoint were not
predetermined in the statistical plan so need further varification.
Secondary end points: There was no effect on average serum sex hormone
analysis: Testosterone 13.4 nmol/l (nr 9-29) FSH 9.2 iu/l (nr 2-12), LH 7.4 iu/l
(nr 2-9) There was no effect on cholesterol, INR or BP amoung men taking
warfarin or ramipril. MRI scans, taken routinely as part of their AS protocol,
were retrospectively evaluated. 35% had no visual disease, 15% had progressive
disease with significant rises in PSA (removed from trial) 50% had visual
disease with no progression, no man remaining on Pomi-T had MRI defined
progression. There was a 14% difference in urinary symptoms (mainly
urgency) and a 6% difference in joint pains although these two endpoint were not
predetermined in the statistical plan so need further varification.

 Late
toxicities such as
Late
toxicities such as Epidemiology
studies have linked higher intake of polyphenol-rich foods with better
Epidemiology
studies have linked higher intake of polyphenol-rich foods with better