Your doctor has recommended a medication called MabThera as treatment for your Non Hodgkins Lymphoma (NHL).
This information sheet provides a brief
introduction to MabThera and explains the common side effects you may experience.
This does not mean you will definitely get them. It is also possible you may
experience a side effect not mentioned here. MabThera
may be given in conjunction with chemotherapy so you should also refer to the
specific information and advice sheet relating to these other drugs.
 What
is Mabhtera? It is not
chemotherapy and it is not a hormone
therapy. It is called a monoclonal antibody (Mab) and is one of the novel new cancer
therapies which utilise the natural immune system (Immunotherapy). Cancers, grow mainly outside the
control of the bodies normal regulatory systems (what is cancer). Despite this they are sufficiently similar to the own bodies
cells to escape the normal bodies defence mechanism against "foreign" attack, as
a result they are able to hide from the bodies immune system. There are, however, some
differences between cancer and normal body cells. These differences can be detected with
sensitive laboratory tests. In some types of NHL a protein is present on the cell
surface (an antigen) called CD20. Over expression (making too much) of
CD20 reflects an abnormality in the DNA
of the cell - a tiny piece of DNA has moved from one chromosome called number 14 to number
18. This is called a translocation(14;18). This moves a cancer producing gene (previously
locked out of harms way) into a position which turns the normal lymphoid cell into a
cancerous lymphoma cell. This oncogene called bcl-2 is responsible for "over
expressing" CD20. In some cases particularly the low grade
follicular type of NHL there are hundreds more CD20 receptors on the cancer cells than the
normal cells. What
is Mabhtera? It is not
chemotherapy and it is not a hormone
therapy. It is called a monoclonal antibody (Mab) and is one of the novel new cancer
therapies which utilise the natural immune system (Immunotherapy). Cancers, grow mainly outside the
control of the bodies normal regulatory systems (what is cancer). Despite this they are sufficiently similar to the own bodies
cells to escape the normal bodies defence mechanism against "foreign" attack, as
a result they are able to hide from the bodies immune system. There are, however, some
differences between cancer and normal body cells. These differences can be detected with
sensitive laboratory tests. In some types of NHL a protein is present on the cell
surface (an antigen) called CD20. Over expression (making too much) of
CD20 reflects an abnormality in the DNA
of the cell - a tiny piece of DNA has moved from one chromosome called number 14 to number
18. This is called a translocation(14;18). This moves a cancer producing gene (previously
locked out of harms way) into a position which turns the normal lymphoid cell into a
cancerous lymphoma cell. This oncogene called bcl-2 is responsible for "over
expressing" CD20. In some cases particularly the low grade
follicular type of NHL there are hundreds more CD20 receptors on the cancer cells than the
normal cells.
 Technology now
exists to make antibodies (the normal chemicals used by the immune system to detect and
attack foreign particles in the body). These antibodies have been made to detect CD20
receptors. The antibody is therefore an anti-CD20 treatment called Rituximab or its commercial
name MabThera. MabThera
is indicated as a single-agent treatment for relapsed or refractory indolent
NHL, and received European approval in March 2002 for the treatment of
aggressive NHL in combination with CHOP chemotherapy.
In September 2004, MabThera was also given a licence for the first line
treatment of Follicular NHL in combination with CVP (Cyclophosphamide,
vincristine and prednisolone) chemotherapy. The drug is known as
Rituxan in the United States, Japan and Canada, and as MabThera in the rest of
the world. Technology now
exists to make antibodies (the normal chemicals used by the immune system to detect and
attack foreign particles in the body). These antibodies have been made to detect CD20
receptors. The antibody is therefore an anti-CD20 treatment called Rituximab or its commercial
name MabThera. MabThera
is indicated as a single-agent treatment for relapsed or refractory indolent
NHL, and received European approval in March 2002 for the treatment of
aggressive NHL in combination with CHOP chemotherapy.
In September 2004, MabThera was also given a licence for the first line
treatment of Follicular NHL in combination with CVP (Cyclophosphamide,
vincristine and prednisolone) chemotherapy. The drug is known as
Rituxan in the United States, Japan and Canada, and as MabThera in the rest of
the world.
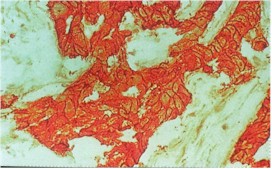 How
does Mabhtera work?
Research has shown that 95% of
patients with low grade follicular lymphoma over express CD20. The higher grades of
NHL are the less likely it is to have CD20 receptors (classification of lymphomas).
In any case, before treatment, this can be measured on a sample of lymphoma in the original biopsy ( a
further biopsy is usually not required). If the NHL does over express CD20 down the
microscope it will stain brightly compared the normal cells (see left
picture). In these patients it is then possible to give MabThera to attack the
lymphoma cells which still remain in the body. In the body the MabThera finds the
cancer cells wherever they may be hiding and sticks to the CD20 receptor. It
then enhances the tumour response in a number of ways:- How
does Mabhtera work?
Research has shown that 95% of
patients with low grade follicular lymphoma over express CD20. The higher grades of
NHL are the less likely it is to have CD20 receptors (classification of lymphomas).
In any case, before treatment, this can be measured on a sample of lymphoma in the original biopsy ( a
further biopsy is usually not required). If the NHL does over express CD20 down the
microscope it will stain brightly compared the normal cells (see left
picture). In these patients it is then possible to give MabThera to attack the
lymphoma cells which still remain in the body. In the body the MabThera finds the
cancer cells wherever they may be hiding and sticks to the CD20 receptor. It
then enhances the tumour response in a number of ways:-
-
It triggers a self destruct mechanism within the cell
(apoptosis).
-
It also encourages
the bodies normal immune cells to attack the tumour.
-
They condition the cancer cell
to be more sensitive to chemotherapy.
 How
is Mabhtera given?
Techniques may vary
but the most commonly used technique is as an infusion into a vein
in the hand or arm through a small plastic tube called a cannula. The infusion rate may
vary on how it is tolerated but on the first occasion it is at least 4-5 hours. This is
usually given as a day case. The dose is calculated by the height & weight of the
patient (then converted into surface area). The recommended dose is 375mg/meter squared
once a week for 4 infusions if given as monotherapy and 8 if given with
chemotherapy. Occasionally, as with all protein based drugs
it is possible to get
an allergic reaction. The nurses will therefore be checking how you are
feeling and measuring your breathing, pulse and blood pressure blood
regularly. Sometimes, in response to mild reaction, it may have to be slowed
down over several hours. Rarely if there allergic reaction is prominent it has
to be stopped altogether. To avoid a mild reaction often paracetamol and an
antihistamine (e.g. piriton) are given before the infusion. How
is Mabhtera given?
Techniques may vary
but the most commonly used technique is as an infusion into a vein
in the hand or arm through a small plastic tube called a cannula. The infusion rate may
vary on how it is tolerated but on the first occasion it is at least 4-5 hours. This is
usually given as a day case. The dose is calculated by the height & weight of the
patient (then converted into surface area). The recommended dose is 375mg/meter squared
once a week for 4 infusions if given as monotherapy and 8 if given with
chemotherapy. Occasionally, as with all protein based drugs
it is possible to get
an allergic reaction. The nurses will therefore be checking how you are
feeling and measuring your breathing, pulse and blood pressure blood
regularly. Sometimes, in response to mild reaction, it may have to be slowed
down over several hours. Rarely if there allergic reaction is prominent it has
to be stopped altogether. To avoid a mild reaction often paracetamol and an
antihistamine (e.g. piriton) are given before the infusion.
 Are
there any side effects?
When
given with chemotherapy the side effect normally relate to the chemotherapy. Mabthera is usually well tolerated.
It has a major advantage over chemotherapy by not damaging the bone marrow. It does have some mild
side effects of its own:- Are
there any side effects?
When
given with chemotherapy the side effect normally relate to the chemotherapy. Mabthera is usually well tolerated.
It has a major advantage over chemotherapy by not damaging the bone marrow. It does have some mild
side effects of its own:-
Infusion
related reaction:-
Fever, chills and
rigors, throat irritation, a runny nose, flushing or pain in the sites of the tumours.
These are usually mild but can be distressing in some patients. They usually occur
30 minutes to 2 hours after starting the infusion and are more common the faster the
infusion . These symptoms can be diminished by taking a paracetamol and an anti-histamine.
The nurse will monitor your pulse and blood pressure during the infusion.
If the reaction
is severe (rare) there may be associated with dizziness, wheezing or a sensation of
throat swelling which may be associated with a drop in blood pressure. In this case, the
infusion will be slowed down or even stopped and the reaction can be
treated with intravenous steroids. Severe infusion reactions are more likely if
there high tumour burden (very advanced bulk disease) In these cases there has very
rarely been fatalities.
Other
potential side effects:-
-
Rarely patients have
experience mild nausea or even vomiting but these only last a few
hours.
-
MabThera does not
damage the heart but if a patient has a pre-existing heart problem such
as heart failure or angina there infusion reaction could make this worse and should be
used in caution.
-
This medicine should only be used during pregnancy where the advantages
to the individual outweigh the potential risks. Seek medical advice from
your doctor.
- There is no information available about the safety of this medicine during
breastfeeding. For this reason, the manufacturer states that it should not
be used during breastfeeding. Seek medical advice from your doctor.
Further general information Your doctors
and specialist nurses are in an ideal position to give you relevant information
on your disease and treatment as they know your individual circumstances. Cancerbackup
has a help line (0808 800 1234) and a prize winning video
available in English, Italian, Urdu, Bengali,
Gujarati & Hindi explaining Radiotherapy & Chemotherapy. Cancernet.co.uk
has over 500 pages describing cancer, its management, practical tips and tool
which patients, their carers and their doctors have found helpful during the
cancer journey.
|


 How
is Mabhtera given?
How
is Mabhtera given?