|
|
||||||||||||||
|
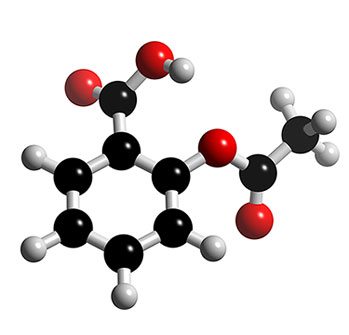
Faslodex (fulvestrant) |
|
|||||||||||||
| Home | Breast cancer | Lifestyle | Treatments | Contact us | ||||||||||||||
Faslodex is indicated for the treatment of postmenopausal women of any age with locally advanced or metastatic breast cancer who have been previously treated with endocrine therapy. It is currently licensed for post menopausal women whether their postmenopausal status occurred naturally or was artificially induced. Faslodex is a competitive oestrogen receptor antagonist, in other words it blocks the effect of oestrogen on cancer cell and hopefully stops them growth and spreading. It is made in the UK by Astrazeneca and used throughout the world,
given as a intramuscular injection once a month. The recommended dose is
500 mg to be administered as a slow intramuscularly once a 1 month as two single 5 mL injections
one in each buttock. Fulvestrant is metabolised primarily in the liver. Caution should be used with Faslodex in patients with creatinine clearance less than 30 mL/min. Caution should be used before treating patients with bleeding diatheses or thrombocytopenia or patients on anticoagulants due to the route of administration. Asthenia has been reported with Faslodex and caution should be observed by those patients who experience this symptom when driving or using machines. Patients with renal insufficiency: No dose adjustments are
necessary for patients with a creatinine clearance greater than 30 mL /min.
Interactions: There are no known drug-drug interactions. Fulvestrant
does not significantly inhibit any of the major cytochrome P450 (CYP)
isoenzymes in vitro, and results from a clinical pharmacokinetic trial
involving co-administration of fulvestrant with midazolam also suggest that
therapeutic doses of fulvestrant will have no inhibitory effects on CYP3A4.
Dosage adjustment is not necessary in patients co-prescribed CYP3A4 inhibitors
or inducers.
FASLODEX™ is a trademark of the AstraZeneca group. SafetyGlide™ is a trademark of Becton Dickinson and Company MORE INFORMATION
|
||||||||||||||